
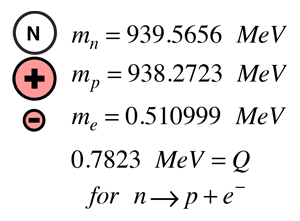
Isotopic substitution can be used to label different parts of the molecules making up a material. the nuclear dependence of scattering allows isotopes of the same element to have substantially different scattering lengths for neutrons.neighbouring elements in the periodic table generally have substantially different scattering cross sections and can be distinguished.it is easier to sense light atoms, such as hydrogen, in the presence of heavier ones.This means that the scattering power (cross-section) of an atom is not strongly related to its atomic number (the number of positive protons in the atom, and therefore number of negative electrons, since the atom must remain neutral), unlike X-rays and electrons where the scattering power increases in proportion to the number of electrons in the atom. Neutrons scatter from materials by interacting with the nucleus of an atom rather than the electron cloud.

Why use neutrons for condensed matter studies? The neutron and the proton form nearly the entire mass of atomic nuclei, so they are both called nucleons.
MASS OF A EUTRON FREE
Neutrons are stable when bound in an atomic nucleus, whilst having a mean lifetime of approximately 1000 seconds as a free particle. Neutron scattering gives detailed information about the microscopic behaviour of condensed matter, playing a major role in shaping the experimental and theoretical understanding of materials ranging from magnetism and superconductivity to chemical surfaces and interfaces.Ī neutron is an uncharged (electrically neutral) subatomic particle with mass 1,839 times that of the electron. The neutron is a powerful tool for the study of condensed matter (solids and liquids) in the world around us, having significant advantages over other forms of radiation in the study of microscopic structure and dynamics.


 0 kommentar(er)
0 kommentar(er)
